
- Precision Weight Based Dosing
- Age and Volume Dosing
- Lower Incremental Dosing
- Convenient and Calculation Free
- BPA Free
- Dishwasher Safe
Dose-2-Weight Syringe®
The Next Generation Liquid Medication Dispenser
The Dose-2-Weight Syringe® is specifically calibrated to the dosage strength of Infants’ Acetaminophen 160/5mL liquid medication and is marked with multiple correlated dosing measurements of child weight, age, and volume of medication. These measurements allow the dispensing of the medication in all recommended dosing methods, including the highest standard of care, by delivering the lowest optimal dose for therapeutic efficacy of 4.57 mg per lb. (10.07 mg per kg) to an exact infant weight. This weight-based dosing method and its optimal dosing of 4.57 mg per lb. is recommended by healthcare professionals and supported by decades of clinical studies; including those utilized for the introduction of Acetaminophen for pediatric use.
This industry unique medication dispenser provides safety and convenience during preparation and administration, while in parallel provides ultra-precise dosing and visual reverification of the amount of medication specific to a child’s age and/or weight. The Dose-2-Weight Syringe® eliminates the need to correlate a specific dose from a medication package or other media to a medication dispenser. This design feature circumvents the procedures shown to be the primary cause of error during the preparation and administration of liquid medications via oral dispenser.
- Measurements For Weight, Age And Volumetric Dosage
- Ultra-Precise Dosing
- Convenient, Ergonomic and Safe
- Calculation Free
- Lower Incremental Indicia
- Easy To Read Markings
- CE/ISO/WHO/TUV Certified
- Short Or Long Tip Barrel
- No Rubber, Latex and BPA Free
- 100% Vision Inspected Calibration Lines
- Dishwasher Safe, Maintains Print Quality
- Multiple Barrel And Plunger Colors Available

84.4% of Parents give their children the wrong dose of medication
DOSING ERRORS ARE THE AMONG THE MOST COMMON AND MOST PREVENTABLE CAUSES OF ADVERSE DRUG EVENTS IN CHILDREN
DOSE-2-WEIGHT SYRINGE® IS THE ONLY ORAL DOSING SYRINGE OFFERING ALL RECOMMENDED OTC DOSING METHODS OF ORAL ACETAMINOPHEN
Health Care Experts Recommend to Dose By Weight or Otherwise Use Age. Now You Can with Our Oral OTC Dispenser
Researchers have shown that only 30% of caregivers are able to demonstrate both an accurately measured and correct dose for their child.¹ Generally over-the-counter (OTC) medications are considered safe, however they are the most frequently implicated pharmaceuticals involved in cases reported to the American Association of Poison Control Center’s National Poison Data System.²
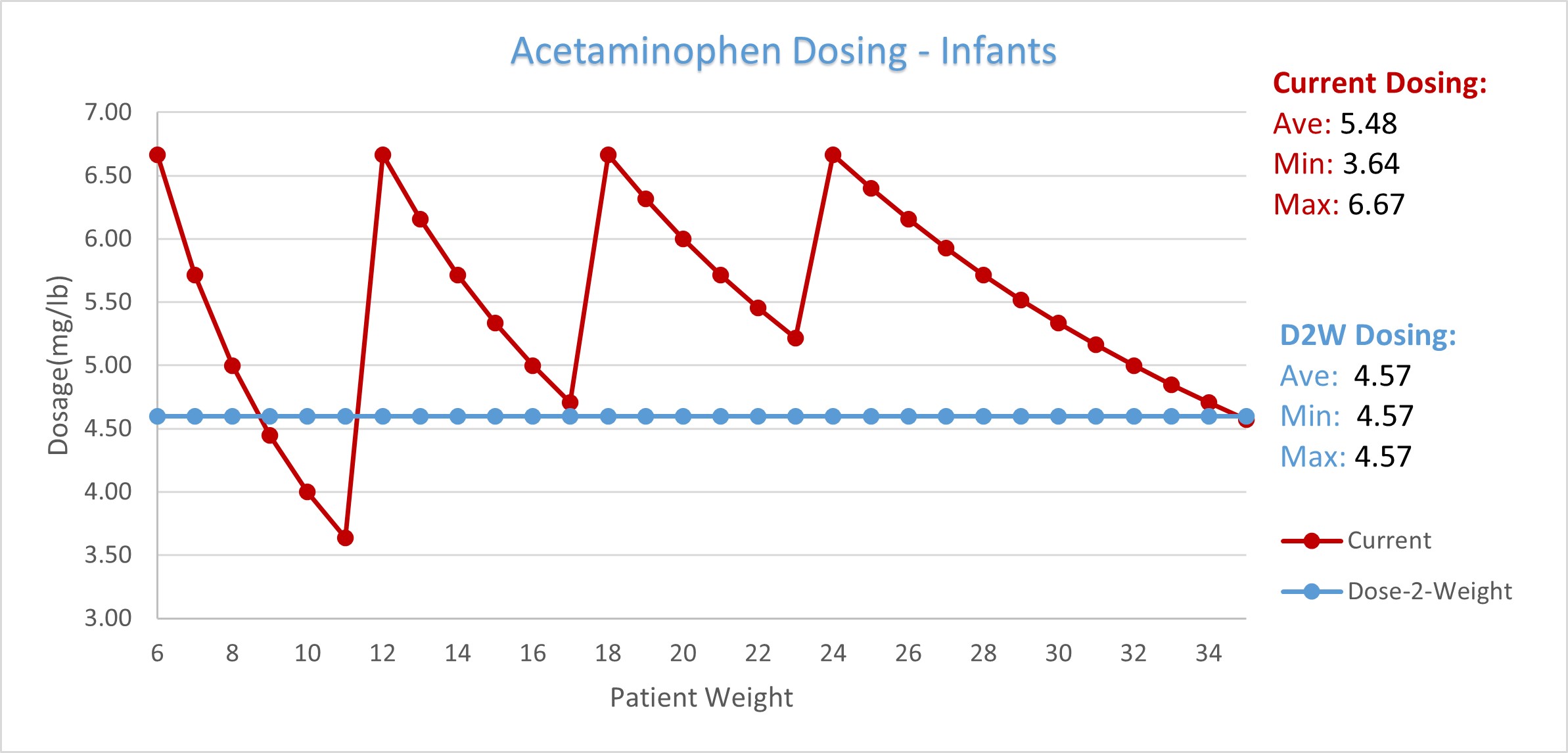
Current dosing recommendations for OTC oral medications commonly specify a volume of medication for a weight or age range, rather than using the dosage strength of the medication specific to an individual patient weight. For example, the current Acetaminophen dosing regimen results with patients in a designated range receiving the same amount of medication, even though their individual weight may differ more than 45% (6-11lbs); and patients in different ranges receiving double the medication (40-80mg) despite having a difference in weight of 1lb, (11-12lbs).
- Simon, H. K., & Weinkle, D. A. (1997). Over-the-counter medications: do parents give what they intend to give? Archives of pediatrics & adolescent medicine, 151(7), 654-656.
- Bronstein AC, Spyker DA, Cantilena LR, Green JL, Rumack BH, Heard SE. 2007 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila). 2008; 46(10):927-1057.
The Most Accurate Way to Dose Infant Tylenol: Meet the Dose-2-Weight Syringe®
When it comes to giving your baby or infant medication, there’s no room for guesswork. Yet, 84.4% of parents unintentionally give their children the wrong dose of medication. That statistic isn’t just alarming—it’s potentially dangerous. One of the most common culprits? Acetaminophen, known to most as Tylenol. That’s why we created the Dose-2-Weight Syringe®—the only oral dosing syringe designed to make infant Tylenol dosage by weight easier, safer, and more accurate.
Precision Matters: Why Weight-Based Dosing is the Standard of Care
When you visit your pediatrician, they don’t ask for your child’s age to determine medication dosage—they ask for your child’s weight. That’s because weight-based dosing delivers the most precise and effective amount of medication, helping to avoid underdosing (which can render the medication ineffective) or overdosing (which can lead to liver damage in the case of acetaminophen).
Our syringe is calibrated to match the 4.57 mg per lb. (10.07 mg per kg) therapeutic dose recommended by leading healthcare professionals. This calculation is built directly into the markings on the syringe—no math required, no hunting through an infant Tylenol dosage chart, and no second guessing.
Designed for Accuracy, Built for Safety
The Dose-2-Weight Syringe® is engineered specifically for use with Infants’ Tylenol (160 mg/5 mL), the most common over-the-counter acetaminophen medication for children. Every detail of our syringe supports a safe, confident dosing experience:
- Multi-method dosing marks : Clearly labeled dosing lines for infant Tylenol dosage by weight, age, and volume, so caregivers can choose the most accurate method available.
- Lower incremental dosing : Provides fine-tuned accuracy at every level, which is essential when dealing with small doses for small bodies.
- Ultra-precise calibration : Every unit is 100% vision-inspected to ensure marking accuracy.
- Easy-to-read labeling : Eliminates visual confusion, even in stressful situations.
- BPA-free, latex-free, rubber-free : Manufactured to exceed safety standards and be compatible with all infants.
- Dishwasher safe : Built for repeated use while maintaining print quality.
- Short and long tip options : Choose the design that works best for your family or clinical practice.
Eliminate Guesswork, Eliminate Risk
Caregivers often rely on the dosing syringe included in the Tylenol box. While trusted, these syringes typically require users to interpret a chart, calculate the right baby Tylenol dosage, and then match it to an imprecise volume line. It’s a system full of opportunities for human error—especially in the middle of the night when your child has a fever and you’re sleep-deprived.
The Dose-2-Weight Syringe® completely bypasses that risk by offering a calculation-free, chart-free experience. Simply find your child’s weight or age and draw the liquid to the corresponding line on the syringe. That’s it.
Even if you don’t know your child’s exact weight, the syringe provides accurate children’s Tylenol dosage by age as a secondary method, making it adaptable to any caregiver’s situation.
A New Standard in Home Medication Dosing
Dosing errors are among the most common and preventable causes of adverse drug events in children. Our goal is to eliminate these preventable errors entirely. We’ve built our product to comply with the most rigorous standards and certifications worldwide:
- CE Certified
- ISO Compliant
- WHO-Recognized Guidelines
- TUV Safety Standards
This isn’t just a convenient tool—it’s a healthcare innovation designed to reduce risk and improve child safety in homes, hospitals, and clinics alike.
Real Problem, Real Solution
It’s important to understand how widespread and serious this issue is. According to the American Association of Poison Control Centers, over-the-counter medications are among the top sources of unintentional poisonings in children, with acetaminophen being one of the most frequent substances involved.
We set out to solve that problem by addressing it at its core: the delivery mechanism. Our syringe is the only product on the market that puts accurate, weight-based acetaminophen dosing into the hands of everyday caregivers.
Whether you're treating an infant’s fever, fussiness, or post-vaccination discomfort, infant Tylenol dosage by weight should never be left to guesswork—and with the Dose-2-Weight Syringe®, it never has to be again.
You don’t need to be a doctor to dose like one. The Dose-2-Weight Syringe® offers the same weight-based precision used in clinical settings, while eliminating the confusion and inaccuracy often found with standard over-the-counter dosing tools. Unlike the typical syringe included with Infants' Tylenol, which only offers volume-based dosing and requires parents to consult a separate infant Tylenol dosage chart, the Dose-2-Weight Syringe® includes built-in markings for weight, age, and volume. This multi-method approach allows caregivers to confidently administer the correct dose without the need for manual calculations.
The markings on the syringe are easy to read and offer lower incremental measurements for finer accuracy—especially important when delivering small doses to infants. Most over-the-counter syringes lack this level of precision and are harder to interpret, increasing the risk of dosing errors. With Dose-2-Weight, that risk is virtually eliminated.
Unlike some syringes that may contain rubber, latex, or BPA, the Dose-2-Weight Syringe® is completely free of these materials, making it a safer choice for babies. It’s also dishwasher safe and maintains its print quality over time, making it practical for repeated use. And because it’s built to clinical-grade specifications and certified by international standards organizations including CE, ISO, WHO, and TUV, it offers a level of reliability unmatched by basic consumer-grade syringes.
Most importantly, the Dose-2-Weight Syringe® drastically reduces the chance of giving too much or too little medication—something that’s all too common when relying solely on age-based dosing or rough volume estimates. Whether you're a first-time parent or a seasoned caregiver, this syringe gives you the confidence and clarity you need to deliver infant Tylenol safely and effectively, every single time.
Who Should Use Dose-2-Weight Syringe®?
- Parents of infants or toddlers
- Pediatricians and nurses
- Daycare providers
- Hospitals and urgent care facilities
- First-time caregivers who want extra peace of mind
No matter your experience level, you deserve a better way to give your child the right dose of medicine. This isn’t just about convenience—it’s about safety, accuracy, and preventing harm.
Dosing Confidence Starts Here
The Dose-2-Weight Syringe® is more than a medical tool—it’s peace of mind. With its FDA-recommended dosing logic, ultra-precise calibration, and caregiver-friendly design, it sets a new gold standard for infant Tylenol dosage.
Because when your child is sick, you should be focused on their comfort—not on doing mental math at 2 a.m.
Let’s stop relying on approximations and start using what works: Tylenol dosage by weight, delivered with unmatched precision by the Dose-2-Weight Syringe®.
Ready to dose smarter?
Choose safety. Choose precision. Choose the Dose-2-Weight Syringe®.

CONTACT US
Asepsis Medical Technologies
1584 Independence Blvd.
Sarasota, FL 34234
United States
Tel: +1 941 360 0029
Fax: +1 941 360 0035
info@asepsismedical.com
Asepsis Medical Technologies
La Polar # 6 Col.
Industrial, CP 07800
Mexico City, Mexico
Tel: +52 (55) 5750 3516
Fax: +52 (55) 2789 5706
Asepsis Medical Technologies
226, Patel Center, Sainath
Road Malad West
Mumbai
Tel: +91 22 2844 2942
Fax: +91 22 6645 9822
Asepsis Medical Technologies
12 F Merrit Industrial
Center Kowloon,
Hong Kong
Tel: +852 9018 2393
Fax: +852 3010 1873





